Covaxin Who Approval Status, Covaxin S Us Approval Delayed As Fda Asks For More Data India News Times Of India
Union Health Minister Mansukh Mandaviya on Tuesday said that approval for Hyderabad-based Bharat Biotechs COVID-19. Due to its pending approval from WHO the European Union and the US are not willing to allow Indians who have been vaccinated by Covaxin to enter their countries.

Who Seeks Additional Clarifications From Bharat Biotech For Covaxin S Emergency Approval Businesstoday
As of now the WHO has approved Covid vaccines by PfizerBioNTech Astrazeneca-SK BioSerum Institute of India AstraZeneca EU.

Covaxin who approval status. Vaccine target product profile. Vaccines under evaluation. New Delhi Oct 29.
Covaxin has been approved by many countries around the world including Australia Greece and Sri Lanka among others. Bharat Biotech had submitted EOI Expression of Interest on 19 April for its COVID-19 vaccine. On arrival tourists and non-residents with proof of a valid negative PCR test result should expect to be required to quarantine for 10 days.
The WHO is currently reviewing the data submitted by Bharat Biotech. However the World Health Organisations approval for the emergency use authorisation EUA to Covid-19 vaccine Covaxin is likely to be delayed as per international public health agency. The African country has approved the made-in-India vaccine Covaxin.
Press Trust of India September 30 2021 104839 IST. An illustration of the Covaxin vaccine. The WHO had on Tuesday said that it will take a call on granting Emergency Use Listing EUL status for Bharat Biotechs COVID-19 vaccine Covaxin next week.
The WHO will take a call on granting Emergency Use Listing EUL status for Bharat Biotechs COVID-19 vaccine Covaxin next week the global health body said on Tuesday. Bharat Biotech Likely to Get WHO Emergency Use Approval For Covaxin on October 5. An emergency approval from the WHO will allow Bharat Biotech to export Covaxin and enable easy international travel of people who have received this vaccine.
The WHO will take a call on granting Emergency Use Listing EUL status for Bharat Biotechs COVID-19 vaccine Covaxin next week the global health body said on Tuesday. WHO to take final call on approval of Covaxin next week By ruchika Published On 2021-10-06T1325230530 Updated On 6 Oct 2021 755 AM GMT New Delhi. WHO said after a meeting on Tuesday that its independent experts had sought additional clarifications from Covaxin maker Bharat Biotech.
Authorities also clarified whether passengers who took the Covishield vaccine would be accepted in the UAE Indian Health Minister Harsh Vardhan holds a dose of Bharat Biotechs COVID-19 vaccine called COVAXIN during a vaccination campaign at All India Institute of. Read all the Latest News Breaking News and Coronavirus News here. The World Health Organisation WHO on Wednesday said it had approved Indian drugmaker Bharat Biotechs COVID-19 vaccine for emergency use paving the way for the homegrown shot to be accepted as a valid vaccine in many poor countries.
WHO to take final decision next week on approval to Covaxin. Additionally people inoculated with Covaxin will be able to travel to many countries without restrictions. Is Covaxin an approved COVID-19 vaccine for travellers from India.
The Technical Advisory Group of the World Health Organisation WHO has recommended Emergency Use Listing status for Bharat Biotechs Covaxin news agency ANI reported on. On delay in approving Covaxin WHO says really trust Indian industry. A series of meetings are taking place to assess pre-clinical and clinical data leading to a crucial decision which is likely by September mid said one of the persons cited above.
Indias Bharat Biotech has been submitting data on the EUL of Covaxin regularly and very quickly to a. The countrys indigenous Covid-19 vaccine has not yet received emergency use approval from WHO. WHO and an independent group of experts are scheduled to meet next week to carry out the riskbenefit assessment and arrive at a decision on granting EUL to Covaxin.
WHOs approval will allow Bharat Biotech to export the vaccine to more countries. WHO seeks additional clarification from Bharat Biotech decision likely on November 3 The technical advisory group will now meet on November 3. October 06 2021 1447 IST.
The Technical Advisory Group of the WHO recommended Emergency Use Listing status for Bharat Biotechs Covaxin after the company submitted a. WHO has published the target product profiles for COVID-19 vaccines which describes the preferred and minimally acceptable profiles for human vaccines for long term protection of persons at high ongoing risk of COVID-19 and for reactive use in outbreak settings with rapid onset of immunity. Foreign Secretary Harsh Vardhan Shringla has said that Bharat Biotechs Covaxin Indias first indigenous Covid-19 vaccine will be approved by the World Health Organisation WHO.
File Photo Australia Monday recognised Indias Covaxin for the purpose of travel to the country as it eased curbs on international travel adding to the list of many other nations which have given their nod to Bharat Biotechs Covid-19 vaccine. WHOs approval for the emergency use authorisation EUA to. WHO to decide on Bharat Biotechs emergency approval for Covaxin in October.
So far only Brazil Iran Philippines Mauritius Mexico Nepal Guyana Paraguay and Zimbabwe have approved the Covaxin. Oman last week and Australia on Monday said they will recognise Covaxin as a valid vaccine for travellers. The status of the assessment for Covaxin is ongoing.
Coronavirus India Approves Covid 19 Vaccines Covishield And Covaxin For Emergency Use The Hindu
Covaxin Here S A List Of Countries That Have Approved The Bharat Biotech Vaccine India News The Indian Express
Diwali Gift For India Bharat Biotech S Covaxin Gets Who Approval

As Indians Who Took Covaxin Await Approval For Travel What S Causing Delay In Who Nod

Covaxin S Us Approval Delayed As Fda Asks For More Data India News Times Of India

Bharat Biotech S Covaxin Likely To Get Who S Emergency Use Approval Today

Bharat Biotech S Covaxin Gets Who Approval For Emergency Use Listing Business Standard News

Covaxin Has Not Got Emergency Use Approval From Who Since 6 Months Know The Process Here
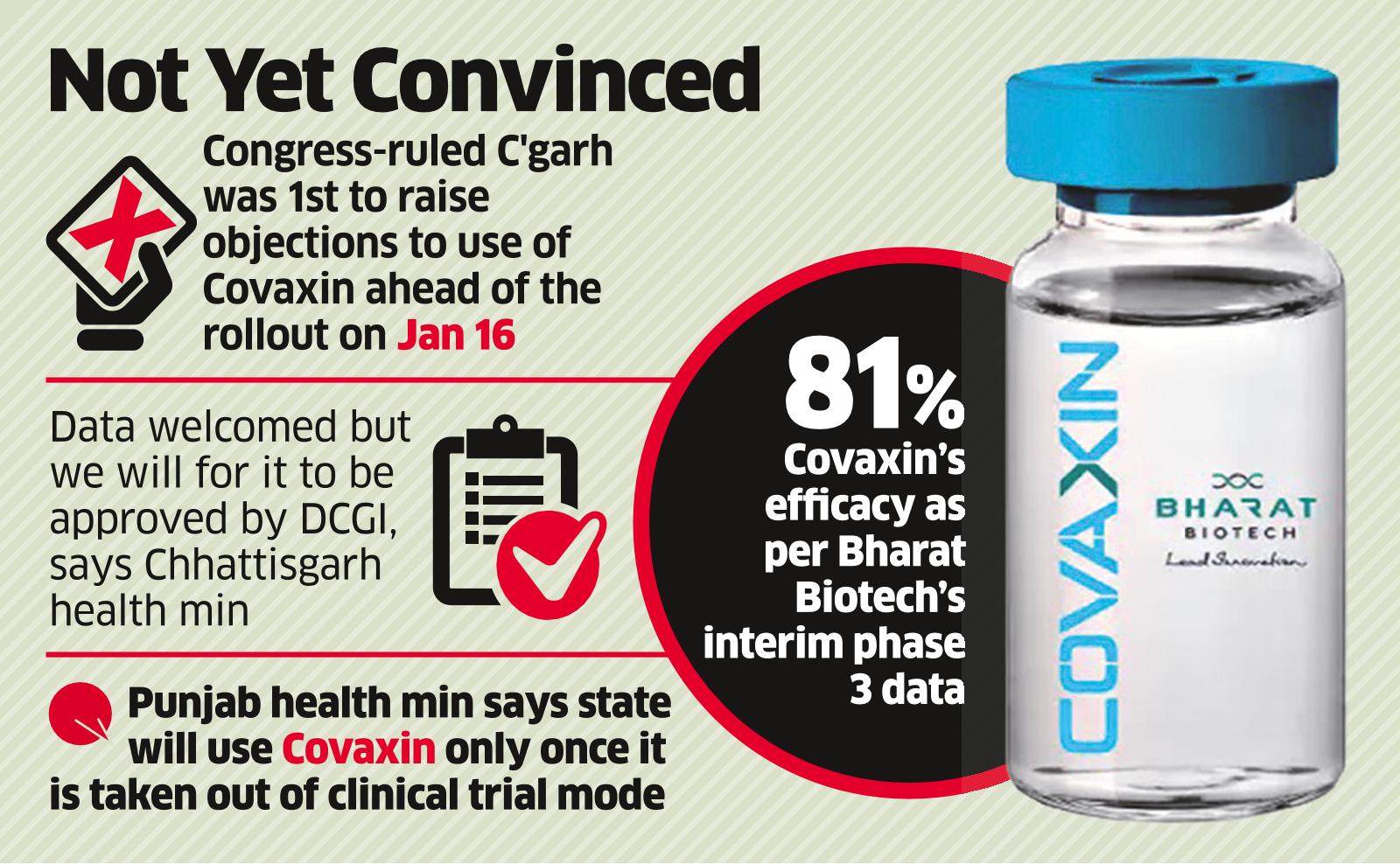
Covaxin Efficacy Data Has No Effect On Opposition States The Economic Times
Covaxin Approval Who Seeks Additional Clarifications For Final Risk Benefit Assessment India News Times Of India

Bharat Biotech S Covaxin Gets Who Approval The Journey So Far

Fact Check Covaxin Has Not Been Approved For Children Above 12 Years Yet Fact Check News
Bharat Biotech S Covaxin Recognised By Australia For Travel India News India Tv

Covaxin Approval Today Crucial W H O Meet Today To Decide On Nod For Bharat Biotech S Covaxin Youtube

Covaxin Who Approval For Travel Status News Update More Updated November 2021 Wego Travel Blog